The intermolecular forces POGIL answer key provides a comprehensive guide to understanding the fundamental forces that govern molecular interactions. By exploring the types, strength, and applications of these forces, we gain invaluable insights into the behavior and properties of substances.
Intermolecular forces, the unsung heroes of chemistry, play a crucial role in shaping the world around us, influencing everything from the melting point of ice to the solubility of drugs. This POGIL answer key unlocks the secrets of these forces, empowering us to unravel the mysteries of molecular interactions.
1. Introduction to Intermolecular Forces
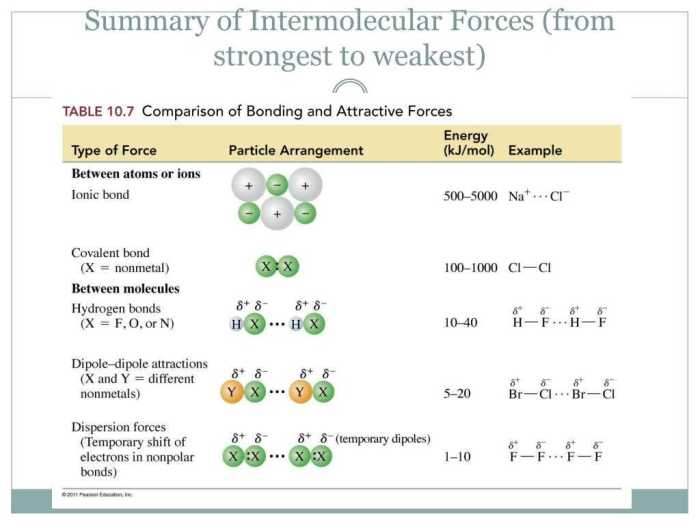
Intermolecular forces (IMFs) are attractive forces that act between molecules. They are weaker than the intramolecular forces that hold atoms together within a molecule. IMFs are important because they determine many of the physical properties of substances, such as their melting point, boiling point, and solubility.
2. Types of Intermolecular Forces: Intermolecular Forces Pogil Answer Key
2.1 Dipole-Dipole Forces
Dipole-dipole forces occur between polar molecules, which have a permanent dipole moment. A dipole moment is a measure of the separation of positive and negative charges within a molecule. The greater the dipole moment, the stronger the dipole-dipole forces.
2.2 Hydrogen Bonding, Intermolecular forces pogil answer key
Hydrogen bonding is a special type of dipole-dipole force that occurs when a hydrogen atom is bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. Hydrogen bonding is stronger than dipole-dipole forces.
2.3 London Dispersion Forces
London dispersion forces are weak attractive forces that occur between all molecules, regardless of their polarity. London dispersion forces are caused by the temporary fluctuations in the electron distribution of a molecule.
3. Strength of Intermolecular Forces

The strength of IMFs depends on several factors:
- Polarity of molecules:The more polar a molecule, the stronger the IMFs.
- Size of molecules:The larger the molecule, the stronger the IMFs.
- Shape of molecules:The more compact a molecule, the stronger the IMFs.
4. Properties of Substances and Intermolecular Forces
IMFs have a significant impact on the properties of substances:
- Melting point:The stronger the IMFs, the higher the melting point.
- Boiling point:The stronger the IMFs, the higher the boiling point.
- Solubility:The stronger the IMFs, the less soluble the substance is in nonpolar solvents.
5. Applications of Intermolecular Forces
IMFs are used in a variety of practical applications:
- Drug design:IMFs can be used to design drugs that bind to specific receptors in the body.
- Food preservation:IMFs can be used to preserve food by preventing the growth of bacteria.
- Separation of materials:IMFs can be used to separate different materials, such as oil and water.
Q&A
What are the different types of intermolecular forces?
The three main types of intermolecular forces are dipole-dipole forces, hydrogen bonding, and London dispersion forces.
How do intermolecular forces affect the properties of substances?
Intermolecular forces influence properties such as melting point, boiling point, and solubility by determining the strength of the interactions between molecules.
What are some practical applications of intermolecular forces?
Intermolecular forces are utilized in drug design, food preservation, and the separation of materials, among other applications.