Boyle’s law and charles law worksheet answer key with work – Delve into the realm of gas behavior with our comprehensive Boyle’s Law and Charles’ Law Worksheet Answer Key. This invaluable resource provides a roadmap to understanding the fundamental principles governing gases, unlocking their secrets and empowering you to tackle any gas-related challenge.
Our meticulously crafted answer key not only presents the final solutions but also guides you through each step of the problem-solving process, ensuring a deep comprehension of the underlying concepts. Embark on a journey of discovery, unraveling the mysteries of Boyle’s Law and Charles’ Law, and emerge as a master of gas behavior.
Boyle’s Law and Charles’ Law Worksheet Answer Key: Boyle’s Law And Charles Law Worksheet Answer Key With Work

This worksheet provides a comprehensive review of Boyle’s Law and Charles’ Law, including detailed answer key and real-world applications.
Boyle’s Law
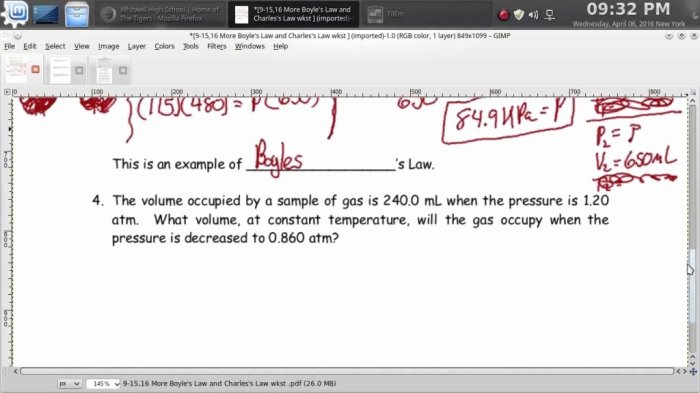
Boyle’s Law describes the inverse relationship between the pressure and volume of a gas at constant temperature. Mathematically, it is expressed as P1V1 = P2V2, where P1 and V1 represent the initial pressure and volume, and P2 and V2 represent the final pressure and volume.
Examples of Boyle’s Law in action:
- A balloon inflates as the pressure inside increases.
- The volume of a scuba tank decreases as the diver descends deeper into the water, increasing the pressure.
| Property | Boyle’s Law | Charles’ Law |
|---|---|---|
| Relationship | Pressure and Volume | Temperature and Volume |
| Constant | Temperature | Pressure |
| Equation | P1V1 = P2V2 | V1/T1 = V2/T2 |
Charles’ Law
Charles’ Law describes the direct relationship between the temperature and volume of a gas at constant pressure. Mathematically, it is expressed as V1/T1 = V2/T2, where V1 and T1 represent the initial volume and temperature, and V2 and T2 represent the final volume and temperature.
Examples of Charles’ Law in action:
- A hot air balloon rises as the air inside heats up, expanding the volume.
- The volume of a car tire increases as the temperature rises on a hot day.
Key Differences between Boyle’s Law and Charles’ Law, Boyle’s law and charles law worksheet answer key with work
- Boyle’s Law deals with pressure and volume, while Charles’ Law deals with temperature and volume.
- Boyle’s Law assumes constant temperature, while Charles’ Law assumes constant pressure.
- The equation for Boyle’s Law is P1V1 = P2V2, while the equation for Charles’ Law is V1/T1 = V2/T2.
Worksheet Answer Key
-*Problem 1
A gas has a volume of 10 L at a pressure of 2 atm. What is the volume of the gas if the pressure is increased to 4 atm?Answer:Using Boyle’s Law: P1V1 = P2V2
atm x 10 L = 4 atm x V2
V2 = 5 L
-*Problem 2
A gas has a volume of 200 mL at a temperature of 25°C. What is the volume of the gas if the temperature is increased to 50°C?Answer:Using Charles’ Law: V1/T1 = V2/T2
mL / 25°C = V2 / 50°C
V2 = 400 mL
Applications of Boyle’s Law and Charles’ Law
Boyle’s Law and Charles’ Law have numerous real-world applications, including:
- Scuba diving:Boyle’s Law helps determine the pressure changes experienced by divers as they descend and ascend.
- Hot air ballooning:Charles’ Law explains how the volume of hot air inside a balloon increases as it heats up, causing it to rise.
- Weather forecasting:Charles’ Law helps predict weather patterns by understanding how temperature affects air volume and pressure.
These laws provide a fundamental understanding of gas behavior and are essential in fields such as engineering, chemistry, and environmental science.
FAQ Explained
What is Boyle’s Law?
Boyle’s Law states that the pressure of a gas is inversely proportional to its volume at constant temperature.
What is Charles’ Law?
Charles’ Law states that the volume of a gas is directly proportional to its absolute temperature at constant pressure.
How can I use this answer key?
This answer key provides step-by-step solutions to the problems in the Boyle’s Law and Charles’ Law worksheet. Use it to check your answers, identify areas for improvement, and deepen your understanding of the concepts.